QIAN Zhuyin1*, ZHANG Bin1, CHEN Yiqiu2, WU Yingchun3, GU Yuqing1, ZHU Yichao2
1Pancreas Center, the Second Affiliated Hospital of Nanjing Medical University, Nanjing 210003; 2Department of Physiology, Nanjing Medical University, Nanjing 211166; 3Ultrasound Department, the Second Affiliated Hospital of Nanjing Medical University, Nanjing 210003, China
[Abstract] Objective: To compare the therapeutic efficiency and risk of intraoperative cryoablation therapy (IOCT) and intraoperative combined cryoablation and hyperthermia (ICCH) in the treatment of unresectable pancreatic cancer. Methods: Patients with unresectable pancreatic cancer admitted to the Pancreatic Center of the Second Affiliated Hospital of Nanjing Medical University were divided into two groups: IOCT group (101 cases) and ICCH group (35 cases). The clinical data of patients were analyzed and surgical methods were compared. The incidence of postoperative complications, survival rate, tumor indexes and pain scores were compared in groups. Results: Compared with IOCT group, the intraoperative blood loss was less in ICCH group (P < 0.01). The postoperative fasting time in ICCH group was shorter than that in IOCT group (P < 0.01). The incidence and severity of postoperative complications in ICCH group were lower than those in IOCT group (P < 0.05). The 1 -year survival rate of patients with pancreatic head carcinoma was significantly higher in ICCH group than in IOCT group (P=0.034). Conclusion: ICCH is more effective in the treatment of unresectable pancreatic cancer, with lower risk and lower complication rate than IOCT.
[Key words] unresectable pancreatic cancer; intraoperative combined cryoablation and hyperthermia; postoperative complications; survival rate
[CLC No.] | R735.9 | [Document code] | A | [Article No.] | 1007-4368(2021)08-1203-05 |
DOI: 10.7655/NYDXBNS20210815
[J Nanjing Med Univ, 2021, 41(08): 1203-1207]
[Fund program] General Topic of Nanjing Health Science and Technology Development Special Fund Program (YKK18188), General Project of Nanjing Medical University Science and Technology Development Fund (NMUB 2019044, NMUB2019045, NMUB2019059, NMUB2019060)
*Corresponding author, E-mail: qianzhusilver@163.com
Pancreatic cancer, known as the "king of cancer", is a highly malignant tumor of the digestive system[1-2]. The incidence of pancreatic cancer is hidden, and early diagnosis is difficult. More than 80% of patients with pancreatic cancer have lost the chance of radical surgery at the time of initial diagnosis. The prognosis of unresectable pancreatic cancer is extremely poor, and the median survival time is only 3-6 months[3]. At present, the traditional treatment of unresectable pancreatic cancer is based on systemic chemotherapy. Patients often cannot tolerate strong chemotherapy regimens, and the effect is poor. Therefore, exploring an effective comprehensive treatment for unresectable pancreatic cancer has become a research hotspot in the field of treatment and prevention of tumor in digestive system.
Intraoperative combined cryoablation and hyperthermia (ICCH) is an improvement and upgrading of the center since 2020 on the basis of the original intraoperative cryoablation therapy (IOCT), which is the first new protocol for the treatment of unresectable pancreatic cancer at home and abroad that integrates deep cryoablation and hyperthermia. This technology innovatively realizes an ultra-wide temperature range from -196°C to 80°C in the same ablation probe, breaking through the limitations of previous single cryotherapy or hyperthermia, and combining the advantages of both. This study summarizes the clinical practice experience of using IOCT and ICCH in the treatment of unresectable pancreatic cancer in the past 3 years and shares it with colleagues.
1.1 Objects
From June 2018 to March 2021, 136 patients who underwent ablation surgery in the Pancreatic Center of the Second Affiliated Hospital of Nanjing Medical University were confirmed as pancreatic cancer by fast frozen section, paraffin section examination and imaging diagnosis. Inclusion criteria: ① locally progressive pancreatic cancer confirmed by imaging and pathological results; ② pancreatic cancer with oligometastasis in liver explored by preoperative imaging and intraoperative ultrasound (≤ 3 metastases); ③ informed consent and signing of informed consent form by patients and family members. Exclusion criteria: ① pancreatic cancer with liver or abdominal cavity widespread metastasis, or distant metastasis; ② pancreatic cystic tumor with malignant transformation, or pancreatic cancer with cystic change or necrosis; ③ patients with severe cardiopulmonary, liver and renal insufficiency or other basic diseases who cannot tolerate general anesthesia and surgery. Based on the factors such as patient age and gender, all patients were divided into two groups according to their propensity score matching: 101 patients in the IOCT group, aged (62.68±8.67) years, including 56 males (55.4%) and 45 females (44.6%); 35 patients in the ICCH group, aged (62.91±9.25) years, including 20 males (57.1%) and 15 females (42.9%). According to the staging criteria for pancreatic cancer (8th edition) of American Joint Committee on Cancer (AJCC) , there were 60 patients in stage III and 41 patients in stage IV in the IOCT group; there were 25 patients in stage III and 10 patients in stage IV in the ICCH group. There was no statistically significant difference in tumor location, tumor size and preoperative complications between the two groups. This study was approved by the medical ethics committee of the Second Affiliated Hospital of Nanjing Medical University (approval No.: 2018-KY-088).
1.2 Methods
Open surgery shall be performed for the both groups. After intraoperative exploration confirmed that the tumor could not be surgically resected, the ultrasound examination of tumor shall be then performed. The tumor size and its adjacent relationship with peripheral organs were judged by ultrasound examination[2], and the tumor needle biopsy was performed under ultrasound guidance. After obtaining the pathological results, IOCT or ICCH was performed first, and then the biliary tract or intestinal obstruction caused by the tumor was relieved.
IOCT group: Accurately puncture the ablation probe with a diameter of 1.7 mm to the farthest end of the tumor under the intraoperative ultrasound guidance, freeze the target tissue to about -150°C within 15 s through argon refrigeration effect, with the freezing time of 8-15 min, meanwhile, flush with warm saline to protect the peripheral organs of the ice ball, rewarm to 30°C through the thermal effect of helium, and regard as 1 cycle; repeat the above treatment for a total of 2 cycles, fully rewarm in sections, and pull out the probe.
ICCH group: Accurately puncture the ablation probe with a diameter of 2.6 mm to the farthest end of the tumor under the intraoperative ultrasound guidance, freeze the target tissue to about -196°C through liquid nitrogen refrigeration effect, with the freezing time of 6-10 min, rewarm to 80°C through the thermal effect of alcohol vapor, and regard as 1 cycle; repeat the above treatment for a total of 2 cycles, fully rewarm in sections, monitor the thawing of ice ball with ultrasound and pull out the probe (Figure 1 and 2).
Whether the shape and size of the ice ball are completely covered the tumor, and the relationship between the ice ball and the peripheral organs were monitored by ultrasound throughout the surgical procedure in both groups, and real-time adjustments were made to avoid frostbite. The diagnosis and grading of complications such as postoperative pancreatic fistula, delayed gastric emptying and postoperative bleeding were defined by the International Study Group for Pancreatic Surgery (ISGPS)[4-6]. Relevant indexes such as carbohydrate antigen 199 (CA199), total bilirubin (TB), albumin (ALB), blood glucose testing, and pain index score (VAS score) were performed respectively before and after the surgery. All patients were treated with chemotherapy according to the conventional regimens after the surgery. Patients with performance status (PS) of 0-1 were treated with AG regimen (Albumin-bound paclitaxel + Gemcitabine), patients with PS of 2 were treated with GS regimen (Gemcitabine + Fluorouracil) or Gem and S1 mono-chemotherapy, and patients with PS of >2 were not included in this study.
1.3 Statistical methods
SPSS 22.0 was used for statistical analysis, the quantitative index of normal distribution were expressed in mean ± standard deviation (x̅±s), and the variance analysis was adopted for comparison among groups; the quantitative data of abnormal distribution were expressed in median (quartile) [M(Q25, Q75)], and the Mann-Whitney U test was adopted for comparison among groups; the qualitative data were expressed in case numbers and constituent ratios, and the chi-square test or Fisher exact probability method was adopted for comparison among groups. The survival curves were drawn by GraphPad Prism 6, and the ordinates were shown as percentages, and the Log-Rank (Mantel-Cox) test was adopted for comparison among groups. The difference P<0.05 is statistically significant.

A: intraoperative ultrasound real-time monitoring; B: ice ball formation can be seen at the tumor.
Figure 1 Open abdomen of patients with unresectable pancreatic cancer in treatment of ICCH

Figure 2 The temperature curve of ICCH for unresectable pancreatic cancer
2.1 Surgical data
Compared with the IOCT group, the ICCH group reduced the intraoperative blood loss (P <0.05, Table 1), which can greatly improve the therapeutic efficiency of pancreatic cancer ablation and reduce the surgical risk. 12 patients (11.9%) in the IOCT group underwent intraoperative blood transfusion; 2 patients (5.7%) in the ICCH group underwent intraoperative blood transfusion. The postoperative fasting time of the ICCH group was shorter than that of the IOCT group, but the postoperative hospital stay was slightly longer than that of the IOCT group (P <0.05, Table 1). In terms of complications, there was no statistically significant difference in the incidence of postoperative pancreatic fistula and delayed gastric emptying between the two groups, but there was no postoperative bleeding or pulmonary infection in the ICCH group (P <0.05, Table 1).
2.2 Perioperative efficacy evaluation
Serum tumor index CA199, TB, ALB, blood glucose testing and VAS score were performed before and after the surgery for control study. The VAS score and CA199 of the two groups decreased significantly after surgery, but the difference between the two groups was small and there was no statistical significance (P >0.05). However, after ablation treatment, the blood glucose in the ICCH group was lower than that in the IOCT group, and the difference was statistically significant (P <0.05, Table 1).
2.3 Evaluation of short-term survival rate of pancreatic head cancer within 1 year after surgery
Due to the short development time of ICCH technology, we only selected the patients with pancreatic head cancer who underwent IOCT or ICCH 1 year after surgery for survival analysis. There were 42 patients in the IOCT group and 11 patients in the ICCH group. The difference in tumor stage between the 2 groups is not statistically significant. The 1-year survival rate of patients with pancreatic head cancer was compared between the two groups. In the ICCH group, 6 patients with the survival period >1 year, 1 patient with the survival period >6 months to 1 year, 3 patients with the survival period ≤ 6 months, and 1 patient with loss to follow-up; In the IOCT group, 6 patients with the survival period >1 year, 9 patients with survival period >6 months to 1 year, 16 patients with survival period ≤ 6 months, and 11 patients with loss to follow-up. The postoperative survival rate of the ICCH group was significantly better than that of the IOCT group (P =0.034, Figure 3).
Pancreatic cancer is still one of the most lethal malignant tumors, with difficult early diagnosis and extremely poor prognosis. The 5-year survival rate is still only 9%. Surgical resection is the only possible treatment for pancreatic cancer. However, the incidence of pancreatic cancer is hidden, and only less than 20% of patients can be resected after diagnosis[7]. At present, gemcitabine based single drug or combination chemotherapy is the main treatment for unresectable pancreatic cancer. Studies have confirmed that FOLFIRI-NOX or nano - paclitaxel combined with gemcitabine can improve the survival rate, but the median survival time is still less than 1 year[8]. Despite the continuous development of chemotherapy regimens and target treatment, pancreatic cancer still shows high resistance to chemotherapy.
In recent years, local treatments such as high-intensity focused ultrasound treatment, irreversible electroporation[9], cryoablation[10-11] and radiofrequency ablation have been gradually carried out in unresectable pancreatic cancer, showing the advantages of relative safety, efficiency and convenience, with significant efficacy in local tumor control and relieve pain[12]. The ideal combined treatment can improve the living quality and survival rate of patients without increasing complications[13]. However, there is no standard combined treatment scheme, which needs further clinical research[14]. This research group has carried out intraoperative IOCT for unresectable pancreatic cancer since January 2017, and has achieved preliminary results. The postoperative tumor indexes CA199 and VAS pain scores decreased significantly, with tumor shrinking, and the postoperative survival time was better than that of traditional chemoradiotherapy[10-11].
Both single thermal ablation and cryoablation have certain deficiencies. Single thermal ablation is used to prevent the spread of high temperature to peripheral healthy tissues, preserving the "safe range" of the peripheral edge in the treatment, and avoiding the ablation of the whole pancreatic tumor, leading to incomplete clearance of the pancreatic tumor, easy recurrence, and often accompanied by complications such as duodenal and major vascular injury and pancreatic fistula[15]. Hyperthermia is often accompanied by an increase in hemoperfusion in the peripheral area of the tumor, which leads to heat loss and lowers the temperature of the tumor edge, weakening the hyperthermia effect. The increased blood flow caused by local hyperthermia also carries the risk of stimulating the metastasis of pancreatic tumors from the primary site[16]. The deficiency of argon-helium cryoablation is that the tumor cells are not completely killed and the recurrence rate of pancreatic tumors is higher. Cryoablation can cause strong inflammation and blood coagulation reactions, or lead to a higher incidence of complications, compared to radiofrequency ablation or laser ablation. In terms of complications, there were 2 patients with postoperative bleeding and 16 patients with pulmonary infection in the IOCT group of this study; however, the ICCH group did not experience any of the above complications. Cryoablation may sometimes cause systemic inflammatory response syndrome, known as "cold shock", which is life-threatening[15].
Table 1 Operation indexes of patients with unresectable pancreatic cancer
Indicator | IOCT group (n =101) | ICCH group (n =35) | χ2/F/Z value | P value |
Surgical method [n (%)] | 0.346 | 0.556 | ||
Argon-helium ablation | 52(51.5) | 16(45.7) | ||
Ablation + bilioenteric / gastrointestinal anastomosis | 49(48.5) | 19(54.3) | ||
Intraoperative blood loss (ml) | 200(100, 200) | 100(50, 200) | -2.555 | 0.011 |
Postoperative fasting time (d) | 5.78 ± 3.39 | 4.31 ± 1.68 | 6.029 | 0.015 |
Postoperative hospital stay (d) | 19.26 ± 8.40 | 20.11 ± 7.00 | 0.002 | |
Vascular invasion [n (%)] | 1.137 | 0.566 | ||
Artery | 28(27.7) | 13(37.1) | ||
Artery and vein | 48(47.5) | 15(42.9) | ||
Vein | 25(24.8) | 7(20.0) | ||
Postoperative complications [n (%)] | ||||
Postoperative pancreatic fistula | 25(24.8) | 7(20.0) | 0.326 | 0.568 |
Delayed gastric emptying | 11(10.9) | 5(14.3) | 0.289 | 0.591 |
Postoperative bleeding | 2(2.0) | 0(0) | 0.703 | 0.402 |
Pulmonary infection | 16(15.8) | 0(0) | 6.284 | 0.012 |
Preoperative TB (μmol/L) | 11.10(7.85, 25.55) | 13.40(8.40, 31.90) | -0.749 | 0.454 |
Postoperative TB (μmol/L) | 13.80(10.45, 27.25) | 15.40(9.48, 36.80) | -0.332 | 0.740 |
Preoperative ALB (μmol/L) | 40.66 ± 4.57 | 39.40 ± 7.96 | 1.300 | 0.256 |
Postoperative ALB (μmol/L) | 33.65 ± 3.95 | 34.48 ± 7.35 | 0.756 | 0.386 |
Preoperative VAS score | 6.66 ± 1.65 | 6.29 ± 1.95 | 1.237 | 0.268 |
Postoperative VAS score | 2.49 ± 1.25 | 2.26 ± 0.82 | 1.018 | 0.315 |
Preoperative CA199 (U/mL) | 331.50(79.37, 1473.00) | 240.90(62.66, 752.20) | -0.189 | 0.850 |
Postoperative CA199 (U/mL) | 178.40(40.65, 1212.00) | 178.30(36.70, 537.20) | -0.162 | 0.871 |
Preoperative blood glucose (mmol/L) | 7.35 ± 3.29 | 6.83 ± 2.70 | 0.696 | 0.406 |
Postoperative blood glucose (mmol/L) | 10.97 ± 2.65 | 9.69 ± 2.04 | 6.751 | 0.010 |
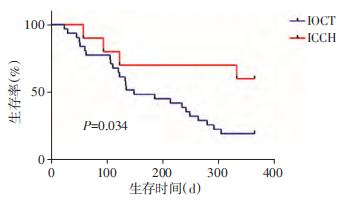
Figure 3 The survival rate of patients with pancreatic head carcinoma within 1 year after surgery
ICCH is the improvement and upgrading based on the single IOCT of this center, and it is the first new protocol for the treatment of unresectable pancreatic cancer at home and abroad that integrates deep cryoablation and hyperthermia[17]. During the surgery of pancreatic cancer, when switching from freezing to heating, intense and rapid temperature rise will produce severe thermal stress and high temperature fracture in the tissue, thus strengthening the damage to pancreatic cancer. Intraoperative combined cryoablation and hyperthermia can achieve complementary advantages of cryoablation / thermal ablation. Hyperthermia can accelerate the rewarming after freezing, causing tumor tissue to undergo drastic temperature changes within a short time, leading to more tumor cell death; meanwhile, it can shorten surgical time and reduce surgical risks. Cryotherapy can reduce blood perfusion in advance, reduce heat loss during hyperthermia, and thus enhance the efficacy of hyperthermia. The hyperthermia effect in intraoperative combined cryoablation and hyperthermia is limited to the range of ice balls, only heating the target tissue, combined with ultrasound real-time monitoring, and stopping after ice balls completely thawed, can effectively avoid thermal injury and ensure the safety of thermal ablation. Both cryotherapy and hyperthermia can inactivate the peripheral nerves at the puncture site, and effect overlay in Intraoperative combined cryoablation and hyperthermia may better relieve pain. In this study, there was no statistically significant difference in preoperative VAS pain score between the IOCT group and ICCH group, and there was no statistically significant difference in postoperative VAS pain scores. This indicates that further research is needed on the impact of postoperative analgesia. Through local cooling and heating, the permeability of tumor vessels is significantly enhanced, and liposomes leak out to the tumor stroma. At the same time, increased thermal stress induces mechanical tissue injury, causing more severe microvascular rupture than simple cryoablation / thermal ablation[18]. Intraoperative combined cryoablation and hyperthermia can make protein denaturation more complete and the ablation zone wider. Hyperthermia can also effectively prevent bleeding and tumor metastasis from the puncture path. In this study, the intraoperative blood loss in the ICCH group was significantly lower than that in the IOCT group. The postoperative blood glucose concentration in the ICCH group was significantly lower than that in the IOCT group, but its clinical significance still needs further research as both groups exceeded normal blood glucose levels.
At present, there is no effective treatment scheme for unresectable pancreatic cancer. The intraoperative combined cryoablation and hyperthermia technology is more effective than argon-helium cryoablation technology in terms of surgical efficiency, tumor control and incidence of complications. Especially for patients with pancreatic head cancer, ICCH treatment can relieve biliary and gastrointestinal obstruction caused by the tumor, obtain pathological diagnosis, and cooperate with postoperative chemotherapy or immunotherapy to further improve living quality and prolong survival time, which is worthy of clinical promotion.
[References]
[1] KLAIBER U, SCHNAIDT ES, HINZ U, et al. Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer[J]. Ann Surg, 2021, 273(1): 154-162
[2]吴迎春,张彬,杨晓俊,等.超声引导技术在术中冷冻消融治疗局部进展期胰腺癌中的应用[J].中国超声医学杂志,2020,36(1):50-52
[3] YOUSAF MN, EHSAN H, MUNEEB A, et al. Role of radiofrequency ablation in the management of unresectable pancreatic cancer[J]. Front Med (Lausanne), 2021, 7: 624997
[4] BASSI C, MARCHEGIANI G, DERVENIS C, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after[J]. Surgery, 2017, 161(3): 584-591
[5] WENTE M N, VEIT J A, BASSI C, et al. Postpancreatec-tomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition[J]. Surgery, 2007, 142(1): 20-25
[6] WENTE M N, BASSI C, DERVENIS C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS) [J]. Surgery, 2007, 142(5): 761-768
[7] MIYASAKA Y, OHTSUKA T, NAKAMURA M. Minimally invasive surgery for pancreatic cancer[J]. Surg Today, 2021, 51(2): 194-203
[8] GIRELLI R, FRIGERIO I, GIARDINO A, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma [J]. Langenbecks Arch Surg, 2013, 398: 63-69
[9] LIU S, QIN Z, XU J, et al. Irreversible electroporation combined with chemotherapy for unresectable pancreatic carcinoma: a prospective cohort study[J]. Onco Targets Ther, 2019, 12: 1341-1350
[10] GU Y, ZHANG B, YANG X, et al. Intraoperative cryoablation of locally advanced pancreatic cancer: report of two cases[J]. Int J ClinExp Med, 2018, 6(11): 6302-6308
[11]张彬,杨晓俊,顾玉青,等.术中冷冻消融在局部进展期胰腺癌治疗中的应用[J].南京医科大学学报(自然科学版),2019,36(5):163-169
[12] D’HAESE J G, HARTEL M, DEMIR I E, et al. Pain sensation in pancreatic diseases is not uniform: the different facets of pancreatic pain[J]. World J Gastroentero, 2014, 20(27): 9154-9161
[13]马洋洋,陈继冰,牛立志.氩氦冷冻消融在肺癌多学科综合治疗中的研究进展[J].介入放射学杂志,2020,29(04):423-428
[14]杨小琴,金成兵.不可切除胰腺癌微无创治疗的研究进展[J].中国癌症防治杂志,2021,13(01):105-110
[15] LINECKER M, PFAMMATTER T, KAMBAKAMBA P, et al. Ablation strategies for locally advanced pancreatic cancer[J]. Dig Surg, 2016, 33: 351-359
[16] LIU K, HE K, XUE T, et al. The cryo-thermal therapy-induced IL-6-rich acute pro-inflammatory response promoted DCs phenotypic maturation as the prerequisite to CD4+ T cell differentiation[J]. Int J Hyperther, 2018, 34(3): 261-272
[17] WU Y, GU Y, ZHANG B, et al. Laparoscopic ultrasonography-guided cryoablation of locally advanced pancreatic cancer: a preliminary report[J]. Jpn J Radiol, 2021.doi: 10.1007/s11604-021-01175-
[18] ZHANG G, LI W, WANG G, et al. Multimode tumor ablation therapy induced different diffusion and microvasculature related parameters change on functional magnetic resonance imaging compared to radiofrequency ablation in liver tumor: an observational study[J]. Medicine (Balti-more), 2020, 99(26): e20795
[Received on] 3/29/2020
 Hygea Medical Technology Co., Ltd.
Hygea Medical Technology Co., Ltd.