
On January 31, 2024, EXCEED™ microwave treatment system and EXACT™ series of microwave ablation probe of Hygea (Beijing) Medical Technology Co., Ltd. successfully obtained permission from the U.S. Food and Drug Administration (FDA), securing a ''pass'' to enter the U.S. market. Based on the application materials approved by the National Medical Products Administration (NMPA) in September 2023, the project team achieved one-time approval from the FDA in an extremely short and efficient manner, from material acceptance to approval. What's even more remarkable is that the microwave products have been approved for use in soft tissues, including a variety of solid tumors. This not only signifies that both products have met international standards in terms of safety, performance, and quality, but also represents a crucial step forward in the international market development for Hygea Medical. Moreover, it marks another key developmental milestone following the initial product, Cryoablation System, receiving European CE certification.

Hygea Medical's microwave ablation products, with their outstanding performance and safety, have successfully passed the rigorous review of the U.S. Food and Drug Administration (FDA) and obtained marketing authorization. From the very beginning of its research and development, this product has adhered to high standards and strict requirements, aiming to set a new benchmark in the market. After numerous experiments and improvements, this high-efficiency and safe medical device was successfully developed. The approval from the U.S. FDA not only demonstrates the leading position of our products in terms of technology and quality, but also signifies high recognition of our company's research and development capabilities and product quality by the international community. FDA review is widely recognized in numerous countries and regions globally, carrying strong authority.

Hygea Medical's President, Luo Fuliang, said: ''After the Cryoablation System obtained the European CE certification in January 2020, the microwave ablation system and ablation probe have also received market approval from the U.S. FDA in early 2024. We are honored to be able to promote the hybrid cold and hot ablation, and thermal ablation of Cryoablation System concurrently to overseas markets, hoping that we can become a business card of Chinese tumor intervention in the international medical device market. Data shows that the market size of microwave system in the United States exceeds $1 billion. In fact, we made many improvements and innovations during the design phase of the microwave ablation machine product. Therefore, microwave ablation carries more expectations for us in the international market.''

The mechanism of microwave ablation is to generate heat through the microwave electromagnetic field, causing local cell and tissue damage. The ablation probe is percutaneously punctured into the tumor tissue, where polar molecules in the tissue rapidly move and rub against each other under the action of the microwave electromagnetic field, producing heat. As the center of the ablation probe reaches a temperature of 120℃~150℃, the proteins of cancer cells undergo complete denaturation and necrosis, thereby achieving the therapeutic goal. Microwave ablation using a single probe generates a spherical or ellipsoidal heat zone with a diameter of 3-5cm. The ellipsoidal heat zone is not conducive to the ablation of small-sized tumors and can cause significant damage to normal tissues. It is more suitable for the treatment of large-sized tumors. The spherical heat zone allows for easier clinical treatment range planning and causes less damage to normal tissues.
Hygea microwave
The microwave ablation project is another key component of Hygea's percutaneous puncture product line, serving as a link between expanding into the international market and penetrating grassroots markets, and providing domestic and foreign oncology experts with a more comprehensive and excellent selection of products.
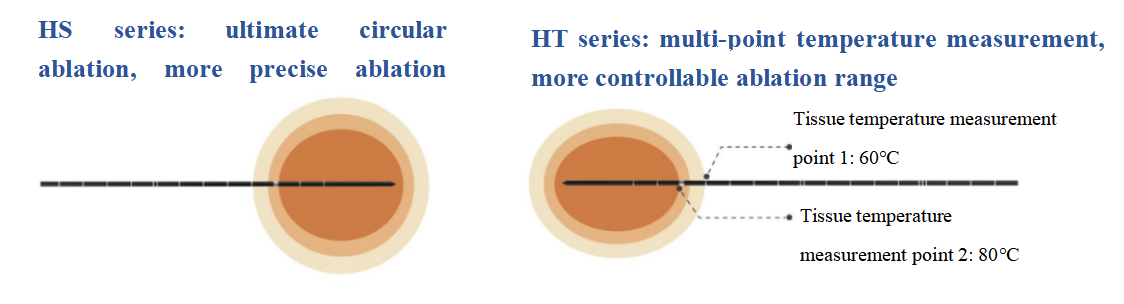
The ablation probe is as fine as 18G (1.3mm) to meet more clinical needs. The full-probe water-cooling design helps reduce tissue carbonization and adhesion. HS series ultimate circular ablation effectively enhances the precision of the ablation range, while HT series multi-point temperature measurement function further increases the controllability of the ablation range. Hygea's fully digital microwave ablation system also features a split-type connecting line, high-end precision peristaltic pump, and innovative dimensions of various control modes.

Currently, the microwave ablation system manufactured in China have not only ''set sail to expand overseas'' but also lead the global development of microwave ablation technology. Hygea's microwave ablation system is a representation of the latest technological innovation in China. Since its launch, it has quickly gained widespread attention at exhibitions such as CCI and CMEF. It is believed that in the future, this system can help the ''Chinese thermal ablation technology family'' gain a larger share in top international terminals and key overseas markets.
Hygea’s Microwave Ablation System, now FDA-approved, offers a minimally invasive treatment for tumors, complementing existing therapies like cryosurgery for lung cancer, cryoablation for bone cancer, cryotherapy for liver cancer, and cryoablation for pancreatic cancer. This advancement provides patients with more options for effective cancer treatment.
 Hygea Medical Technology Co., Ltd.
Hygea Medical Technology Co., Ltd.