QIAN Zhuyin1*, YU Jianhe2, CHENG Zhi3, CHEN Yiqiu4, ZHU Yichao4
1Pancreas Center, the Second Affiliated Hospital of Nanjing Medical University, Nanjing 210003;2Department of Medical Oncology, Xinghua City People’s Hospital, Xinghua 225700; 3ICU, the Second Affiliated Hospital of Nanjing Medical University, Nanjing 210003; 4Department of Physiology, Nanjing Medical University, Nanjing 211166, China
[Abstract] Objective: This study aims to compare the therapeutic effects of ablation therapy combined with chemotherapy and chemotherapy alone in the treatment of stage IV pancreatic cancer, and to explore the potential cytological mechanism of ablation therapy combined with chemotherapy. Methods: Patients with stage IV pancreatic cancer admitted to the Second Affiliated Hospital of Nanjing Medical University were divided into two groups: chemotherapy alone group (chemotherapy group, 13 cases) and ablation therapy combined with chemotherapy group (ablation group, 14 cases). The clinical data of patients were analyzed. The tumor indexes, routine blood indexes, and survival rate were compared between these groups. To study the role of ultralow temperature on the permeability of pancreatic cancer cells, the permeability assay of pancreatic cancer cells was set up based on Boyden chambers. Results: The postoperative total bilirubin (TB) in ablation group was lower than that in chemotherapy group, while the postoperative albumin (ALB) in ablation group was higher than that in chemotherapy group (P < 0.01). The levels of CA19⁃9 in two groups had no significant difference. The counts of both pre-treatmental and after-treatmental white blood cells and the percentages of neutrophils in ablation group were less than those in chemotherapy group (P < 0.05). The count of pre-treatmental platelet in ablation group was more than that in chemotherapy group (P < 0.05). In chemotherapy group, the count of after-treatmental red blood cells was less than the count of pre-treatmental red blood cells (P < 0.01). In ablation group, the percentage of after-treatmental neutrophils was higher than that of pre - treatmental neutrophils (P < 0.01). The survival of patients in ablation group was significantly longer than that in chemotherapy group (P=0.019). The permeability of pancreatic cancer cells was increased after the treatment of ultralow temperature. Conclusion: The patients with stage IV pancreatic cancer have longer survival rates after the treatment of ablation therapy combined with chemotherapy.
[Key words] stage IV pancreatic cancer; cryoablation; chemotherapy; survival; permeability
[CLC No.]: R735.9 | [Document code] A | [Article No.] 1007⁃4368 (2022) 06⁃849⁃05 |
DOI: 10.7655/NYDXBNS20220613
[J Nanjing Med Univ, 2022, 42(06):849⁃853]
__________
[Fund Program] Medical Research Guidance Program of Jiangsu Commission of Health (Z2021021)
∗Corresponding author, E-mail: qianzhusilver@163.com
Pancreatic cancer is a common gastrointestinal tumor with insidious onset, rapid progress, early metastasis and the worst prognosis among all cancers, and the incidence is rising [1]. Distant metastases are often detected during diagnosis, thus losing the opportunities for surgery. For the patients with stage IV pancreatic cancer, gemcitabine-based systemic chemotherapy is a preferred choice at present. However, the traditional chemotherapy alone has poor curative effect, with a median survival time of less than half a year and such therapy is often complicated with serious adverse reactions [1].
Since 2017, the center has tried to treat unresectable advanced pancreatic cancer using physical ablation, and carried out intraoperative pancreatic cancer cryoablation technology in a large scale (intraoperative cryoablation therapy) [2]. After that, the center has achieved an innovation in combining the simple cryoablation technology with high temperature hyperthermia, and launched the pancreatic cancer intraoperative combined cryoablation and hyperthermia (ICCH) since 2019, which can further improve the ablation effect while maintaining the advantages of accurate and safe cryoablation and combining the advantages of hyperthermia [3].
In this study, we compared and analyzed the cases of stage IV pancreatic cancer treated with intraoperative ablation and chemotherapy and the cases of stage IV pancreatic cancer treated with chemotherapy alone, in order to further explore the effect of ablation technology in the treatment of stage IV pancreatic cancer and evaluate its clinical practical value. Moreover, the possible reasons for the effective inhibition of pancreatic cancer cell proliferation by ablation were preliminarily discussed at the cellular level, thus providing a reference for more follow-up studies.
1.1 Objects
27 patients with stage IV pancreatic cancer who met the inclusion and exclusion criteria were admitted to the Second Affiliated Hospital of Nanjing Medical University from June 2018 to June 2021, and were diagnosed as stage IV pancreatic cancer according to the pancreatic cancer staging criteria of American Joint Committee on Cancer (AJCC) (8th Edition). Among them, 13 patients received chemotherapy alone and 14 patients received ablation surgery. Inclusion criteria: patients with stage IV pancreatic cancer were confirmed by imaging and/or pathology results; patients and family members were given informed consents and had signed informed consents. Exclusion criteria: Pancreatic cystic tumor with malignant transformation, or pancreatic cancer with cystic transformation or necrosis; patients who suffer from severe heart and lung, liver and kidney dysfunction or other basic diseases and cannot tolerate general anesthesia and surgery; performance status > 2 points.
After routine chemotherapy, patients made their own informed choice of whether to use ablation (Figure 1). All the cases were divided into two groups: 13 cases for the chemotherapy group, age (58.92±15.09) years old; 14 cases for the ablation group, age (61.86±8.13) years old. There was no statistically significant difference in tumor location, tumor size and preoperative complications between the two groups. This study was approved by the medical ethics committee of the Second Affiliated Hospital of Nanjing Medical University (approval No.: 2018-KY-088)
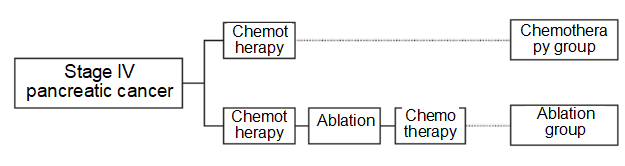
Figure 1 The treatment strategies of chemotherapy group and ablation group
1.2 Methods
1.2.1 Chemotherapy regimen for stage IV pancreatic cancer
Patients in the chemotherapy group were treated with routine chemotherapy regimens based on gemcitabine or albumin-bound paclitaxel. The peripheral blood of the patients in the chemotherapy group was collected on the 7th day after the first round of chemotherapy.
1.2.2 Ablation regimen for stage IV pancreatic cancer
The patients were given preoperative chemotherapy and then ablation therapy. Follow-up chemotherapy was given after surgery with reference to the patient’s performance status (Figure 1). Open surgery shall be performed for the group of patients for ablation. After intraoperative exploration confirmed that the tumor could not be surgically resected, the ultrasound examination of tumor shall be then performed. The tumor size and its adjacency with peripheral organs were judged by ultrasound examination, and the tumor needle biopsy was performed under ultrasound guidance. After obtaining the pathological results, IOCT or ICCH was performed first, and then the biliary tract or intestinal obstruction caused by the tumor was relieved. The peripheral blood of the patients in the ablation group was collected on the 7th day after ablation.
IOCT: Accurately puncture the ablation probe with a diameter of 1.7 mm to the farthest end of the tumor under the intraoperative ultrasound guidance, freeze the target tissue to about -150°C within 15 s through argon refrigeration effect, with the freezing time of 8-15 min, meanwhile, flush with warm saline to protect the peripheral organs of the ice ball, rewarm to 30°C through the thermal effect of helium, and regard as 1 cycle; repeat the above treatment for a total of 2 cycles, fully rewarm in sections, and pull out the probe.
ICCH: Accurately puncture the ablation probe with a diameter of 2.6 mm to the farthest end of the tumor under the intraoperative ultrasound guidance, freeze the target tissue to about -196°C through liquid nitrogen refrigeration effect, with the freezing time of 6-10 min, rewarm to 80°C through the thermal effect of alcohol vapor, and regard as 1 cycle; repeat the above treatment for a total of 2 cycles, fully rewarm in sections, monitor the thawing of ice ball with ultrasound and pull out the probe.
The diagnosis and classification of postoperative complications such as pancreatic fistula, delayed gastric emptying, and postoperative bleeding were defined by the International Study Group of Pancreatic Surgery (ISGPS) [4-6], and carbohydrate antigen 19-9 (CA19-9), total bilirubin (TB), albumin (ALB), blood glucose and other related indicators were detected.
1.2.3 Pancreatic cancer cell permeability test
MIA PaCa-2 human pancreatic cancer cells were routinely cultured in DMEM medium containing 10% fetal bovine serum and penicillin/streptomycin resistance in a 37°C 5% CO2 cell incubator. After digestion and centrifugation of pancreatic cancer cells, the original culture solution was discarded, and the cells were seeded in a Boyden chamber with a pore size of 8.0 μm. The chamber membrane was divided into upper and lower chambers, with a layer of matrigel-coated polyethylene membrane in the middle. 500 μL of culture solution containing 10% fetal bovine serum was added to the lower chamber of the Boyden chamber, and 200 μL of cell suspension was added to the upper chamber to avoid generation of air bubbles, and then the culture was continued in the incubator overnight. In the group with frozen cells, the culture solution in the Boyden chamber was discarded, and liquid nitrogen was quickly added to the upper chamber to freeze the cells. After the liquid nitrogen was completely volatilized, the culture solution containing 10% fetal bovine serum was added. At the same time, the culture solution in the Boyden chamber of the control group was discarded and replaced with the fresh culture solution containing 10% fetal bovine serum. Both groups of cells were cultured for 2 h, the culture medium in the Boyden chamber was discarded, the same volume of phosphate buffered saline (PBS) was added, and 0.1% crystal violet was added to the upper chamber. The liquid in the lower chamber was collected at 1st, 3rd and 5th min after the addition of crystal violet, respectively, and the absorbance value at 590 nm was detected.
1.2.4 Cell viability test
MIA PaCa-2 human pancreatic cancer cells were inoculated into the upper chamber of Boyden chamber in the same as shown in Article 1.2.3. The cells in the group with frozen cells were frozen in liquid nitrogen for 3 min. The cells in the control group were cultured normally. Fresh culture solution containing 10% fetal bovine serum was quickly added or provided and poured in a 37°C 5% CO2 cell culture incubator for routine culture for 48 h. After that, cell viability was detected using a CCK-8 detection kit (Suzhou Meilun Bio). Discard the original culture medium, add the medium containing 10% CCK-8 enhancement solution, continue to culture for another 4 h, and use spectrophotometer to measure the absorbance at 450 nm.
1.3 Statistical methods
SPSS 23.0 was used for statistical analysis, the quantitative data of normal distribution was expressed in mean ± standard deviation ( x ± s), and the variance analysis was adopted for comparison among groups; the quantitative data of abnormal distribution were expressed in median (quartile) [M(P25, P75)], and the Mann-Whitney U test was adopted for comparison among groups. The survival curves were drawn using GraphPad Prism 9, and the Log-Rank (Mantel-Cox) test was adopted for comparison among groups. The difference P<0.05 is statistically significant.
2.1 Evaluation on clinical indicators of chemotherapy and ablation in the patients with stage IV pancreatic cancer
The test results showed that TB in the ablation group was significantly lower than that in the chemotherapy group [14.3(8.9, 18.7) μmol/L vs. 105.9 (19.1, 248.0) μmol/L, Z =-3.106,P = 0.002], while ALB was significantly higher than that in the chemotherapy group [33.6 (30.7, 63.8) μmol/L vs. 29.7 (27.1, 31.1) μmol/L, Z =-2.718, P = 0.007]. The difference in CA19-9 between the two groups before and after treatment was not statistically significant, the number of white blood cells and the percentage of neutrophils before and after treatment in the ablation group were lower than those in the chemotherapy group (P<0.05), and the number of platelets before treatment in the ablation group was higher than that in the chemotherapy group (P<0.05, Table 1).
Table 1 The comparison of the indexes of patients with stage IV pancreatic cancer between chemotherapy and ablation group before and after treatment
[M(P25, P75)]

Compared with the chemotherapy group before treatment, *P<0.05; compared with the chemotherapy group after treatment, #P<0.05.
Besides, we analyzed the differences of blood routine indexes before and after treatment in the chemotherapy group and the ablation group respectively. The number of red blood cells in the chemotherapy group after treatment was significantly lower than that before treatment (P<0.01), and the percentage of neutrophils in the ablation group after treatment was significantly higher than that before treatment (P<0.01, Table 1).
2.2 Evaluation on the survival period of patients with stage IV pancreatic cancer

Figure 2 The survival rate of patients with stage IV pancreatic cancer
The survival period of patients with stage IV pancreatic cancer was analyzed. In the ablation group, there were 4 cases with the survival period longer than 1 year, 3 cases with the survival period longer than 6 months but shorter than 1 year, 6 cases with the survival period shorter than 6 months and 1 case lost to follow up; in the chemotherapy group, there was 1 case with the survival period longer than 1 year, 2 cases with the survival period longer than 6 months but shorter than 1 year and 10 cases with the survival period shorter than 6 months. Survival was significantly better in the ablation group than in the chemotherapy group (P = 0.019, Figure 2).
2.3 Deep cryogenic treatment has improved the permeability of pancreatic cancer cells
The reason for the better curative effect in the ablation group may be related to the permeability of chemotherapeutic drugs in pancreatic cancer tissues. In order to explore the effect of deep cryogenic treatment on the permeability of pancreatic cancer cells at the cellular level, the experimental model of pancreatic cancer cell permeability was constructed on the basis of Boyden chamber. The colorimetric results showed that the dye passing through the polyethylene film increased significantly at the 3rd min after the addition of crystal violet, which was significantly higher than that at the 1st min (P<0.01, Figure 3A). Therefore, the pancreatic cancer cell permeability test used the time point of the 3rd min to detect the difference in permeability between frozen cells (in the group with frozen cells) and non-frozen cells (control group). The absorbance value of the group with frozen cells was significantly higher than that of the control group (P<0.01, Figure 3B), indicating that the permeability of pancreatic cancer cells was significantly enhanced after deep cryogenic treatment. The CCK⁃8 assay detected cell viability and found that the viability of pancreatic cancer cells did not change significantly after rapid deep cryogenic treatment (P>0.05, Figure 3C). We speculated that deep cryogenic treatment improves the permeability of chemotherapeutic drugs in pancreatic cancer tissues.
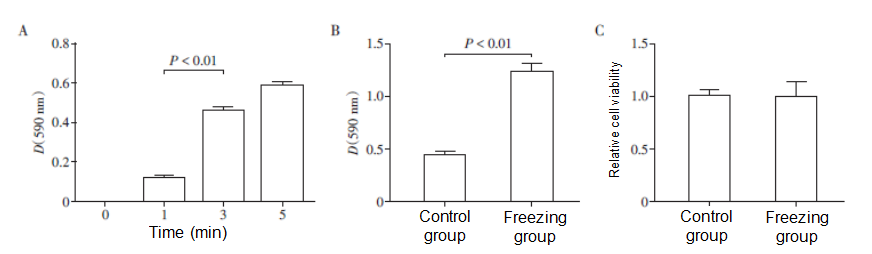
A: Establishment of experimental model of MIA PaCa⁃2 human pancreatic cancer cell permeability; B: Effect of deep cryogenic treatment on the permeability of pancreatic cancer cells; C: Viability of pancreatic cancer cells after deep cryogenic treatment (n = 5).
Figure 3 The ultralow temperature improves the permeability of pancreatic cancer cells
Pancreatic cancer is highly malignant and has a poor prognosis. At present, radical surgical resection is the only effective way for patients with pancreatic cancer to obtain a chance of recovery and long-time survival. Pancreas has a special anatomical location and biological characteristics. Therefore, pancreatic cancer has an insidious onset and it is difficult to detect such cancer in the early stage. 80% of patients have lost the opportunity of surgery during diagnosis, and have a poor sensitivity to chemotherapy and radiotherapy, so no targeted drug is available at present. The efficacy of a single treatment is not satisfactory, and it is difficult to break through the efficacy in the short term. With the popularization of combined cancer treatments in recent years, the multidisciplinary diagnosis and treatment model has gained an increasing popularity in clinical practice, and has achieved remarkable results in the treatment of pancreatic cancer [7]. Reasonable combination in different disease stages can avoid adverse reactions while maximizing the curative effect, help formulate reasonable treatment regimens by fully evaluating the condition of patients, and further improve the overall prognosis of patients with pancreatic cancer [8].
In this study, patients with stage IV pancreatic cancer in the ablation group were treated with routine chemotherapy before surgery, and then ablation therapy was performed to inactivate the primary tumor to the greatest extent, and at the same time, the biliary tract or intestinal obstruction caused by the tumor was relieved. According to the performance status of patients, follow-up chemotherapy was given to inhibit the residual tumor tissues, prevent postoperative recurrence and prolong the survival period of patients. It also conforms to the principle of multidisciplinary comprehensive treatment, greatly improves the curative effect, prolongs the survival period of patients, and strengthens the life quality of patients.
At present, systemic chemotherapy is mostly used for the patients with unresectable pancreatic cancer, and the first-line drugs include gemcitabine-based monotherapy or combination therapy, in which the combination therapy of albumin-bound paclitaxel and gemcitabine is more common. Recent studies have shown that the FOLFIRINOX regimen can extend the median survival period to 13 months, but this improvement is accompanied by an increase in toxicity and adverse effects, making it only suitable for patients with good performance status [9]. Several studies have demonstrated that fluoropyrimidine-based chemotherapy can improve median survival period and disease-free survival period [10]. However, pancreatic cancer tissue is composed of dense fibrous tissues, which can lead to vascular collapse. And the tissue continues to be in a state of hypoxia and ischemia [11], the blood flow is smaller, chemotherapy drugs are difficult to penetrate such tissue and get absorbed, chemotherapy sensitivity is very low, the curative effect is not good, and the treatment is often accompanied by intolerable adverse reactions. For advanced pancreatic cancer, chemotherapy alone cannot meet the needs of prolonging survival period and improving life quality. Under such circumstance, multidisciplinary comprehensive treatment is needed to achieve better effects.
In this study, the experimental model of human pancreatic cancer cell permeability was established by using Boyden chamber to explore the effect of deep cryogenic treatment on the human pancreatic cancer cell permeability. The results showed that the pancreatic cancer cell permeability after deep cryogenic treatment was significantly enhanced. Moreover, we found that rapid deep cryogenic treatment did not affect the viability of pancreatic cancer cells. Cells cryopreserved by liquid nitrogen have been widely used in scientific research and clinical practice, so the results of the above cell viability experiments have met scientific expectations. However, there was no experimental evidence on the effect of rapid deep cryogenic treatment on tissues (especially living tissue) in this study. Rapid freezing in ablation may make subsequent chemotherapy drugs more likely to enter the tumor tissue, improve the low sensitivity of pancreatic cancer to chemotherapy [12], achieve a synergistic pro-apoptotic effect and comply with the principle of multidisciplinary integration.
Inflammatory cells may stimulate the proliferation or metastasis of tumor cells by affecting the tumor microenvironment [13]. Increased numbers of neutrophils were observed in both peripheral blood and tumor tissues of patients with different types of cancer. Neutrophils play a crucial role in tumor metastasis, which will promote angiogenesis and tumor proliferation, and trigger immunosuppression [14]. Furthermore, neutrophil and white blood cell levels are associated with pancreatic cancer prognosis [15]. In this study, the white blood cells and neutrophils after treatment were significantly lower in the ablation group than in the chemotherapy group, and the levels in the chemotherapy group were higher than normal. Lower neutrophil and white blood cell levels after ablation therapy may predict a better prognosis and longer survival period.
At present, there is still a lack of effective chemotherapy drugs for stage IV pancreatic cancer, and the complications of chemotherapy are serious. Intraoperative ablation combined with follow-up chemotherapy shows better efficacy in prolonging survival period and improving life quality, which is worthy of clinical promotion.
[References]
[1]曹毛毛,陈万青.中国恶性肿瘤流行情况及防控现状[J].中国肿瘤临床,2019,46(3):145-149
[2]张彬,杨晓俊,顾玉青,等.术中冷冻消融在局部进展期胰腺癌治疗中的应用[J].南京医科大学学报(自然科学版),2019,39(5):163-169
[3]钱祝银,张彬,陈奕秋,等.术中冷冻消融和冷热复合消融治疗不可切除胰腺癌的临床研究[J].南京医科大学学报(自然科学版),2021,41(8):1203-1207
[4] BASSI C, MARCHEGIANI G, DERVENIS C, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after [J]. Surgery, 2017,161(3):584-591
[5] WENTE M N, VEIT J A, BASSI C, et al. Postpancreatec-tomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition[J]. Surgery, 2007,142(1):20-25
[6] WENTE M N, BASSI C, DERVENIS C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS) [J]. Surgery, 2007,142(5):761-768
[7] 王成锋,杨尹默,傅德良.中国胰腺癌多学科综合治疗模式专家共识(2020版)[J].临床肝胆病杂志,2020,36(9):1947-1951
[8] STROBEL O, NEOPTOLEMOS J, JAGER D, et al. Optimizing the outcomes of pancreatic cancer surgery [J]. Nat Rev Clin Oncol, 2019,16(1):11-26
[9] GROSSBERG A J, CHU L C, DEIG C R, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma [J]. CA Cancer J Clin, 2020,70(5):375-403
[10] FOGEL E L, SHAHDA S, SANDRASEGARAN K, et al. A multidisciplinary approach to pancreas cancer in 2016: a review [J]. Am J Gastroenterol, 2017,112(4):537-554
[11] LONGO V, BRUNETTI O, GNONI A, et al. Angiogenesis in pancreatic ductal adenocarcinoma: a controversial issue [J]. Oncotarget, 2016,7(36):58649-58658
[12] GAGE A A, BAUST J M, BAUST J G. Experimental cryosurgery investigations in vivo [J]. Cryobiology, 2009,59(3):229-243
[13] RAPOPROT B L, STEEL H C, THERON A J, et al. Role of the neutrophil in the pathogenesis of advanced cancer and impaired responsiveness to therapy [J]. Molecules, 2020,25(7):1618
[14] ZHOU B, DENG J, CHEN L, et al. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors [J]. Sci Rep, 2017,7(1):17506
[15] DENG G C, YAN H, GUO Z P, et al. Correlation between baseline serum tumor markers and clinical characteristic factors in patients with advanced pancreatic cancer [J]. Onco Targets Ther, 2020,13:11151-11163
[Received on] January 9, 2022
(Editor of this article: Jiang Li)
 Hygea Medical Technology Co., Ltd.
Hygea Medical Technology Co., Ltd.